
In this paper are entangled with those debates. For this reason, they have figured prominently in recent debates on scientific realism. Their centrality to past scientific practice, however, several of them (e.g., phlogiston, caloric, and the ether) turned out Hidden entities have played a crucial role in the development of the natural sciences. Conversely, I suggest that the history of those entities has important lessons to teach to Issues raised by “hidden entities”, entities that are not accessible to unmediated observation, can enrich the historical In this chapter I investigate the prospects of integrated history and philosophy of science, by examining how philosophical Among those phenomena were a “light which appears about the negative electrode” and a fluorescence in the glass of the tube (, pp.

He observed various complex and striking phenomena associated with the discharge. In 1857 Geissler's tubes were employed by Julius Plücker (1801–1868) to study the influence of a magnet on the electrical discharge. In 1855 the German instrument maker Heinrich Geißler (1815– 1879) manufactured improved vacuum tubes, which made possible the isolation and investigation of cathode rays.

Below a certain pressure the glow assumed a stratified pattern of bright and dark bands.ĭuring the second half of the nineteenth century the discharge of electricity through gases became a topic of intense exploratory experimentation, primarily in Germany. By the middle of the nineteenth century it was known that the passage of electricity through a partly evacuated tube produced a glow in the gas, whose color depended on its chemical composition and its pressure. The latter phenomenon had been studied since the early eighteenth century. (The term electron was not completely new it had been invented in 1891 for the rays themselves.) Thomson was thus the first to discover that particles smaller than atoms existed, and for his pioneering work he was awarded the 1906 Nobel Prize for physics.The detection of cathode rays was a by-product of the investigation of the discharge of electricity through rarefied gases. The particles, first named corpuscles, were later called electrons.

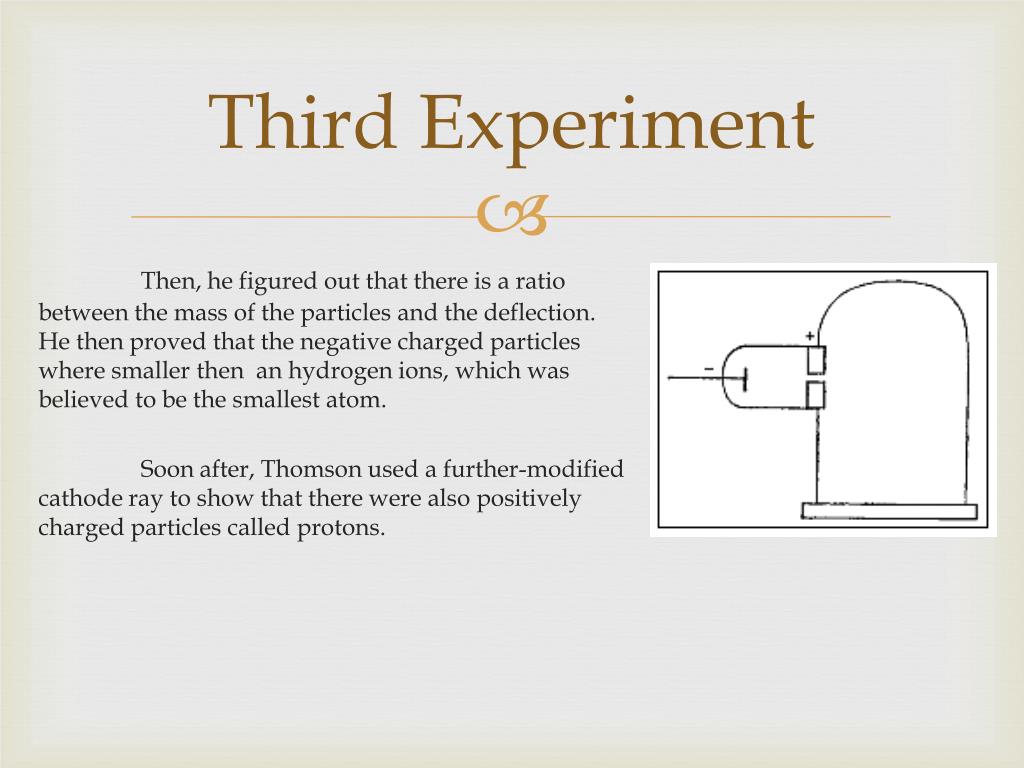
By examining both the energy of the rays and the amount by which an electric charge deflected them, Thomson was able to calculate that these particles had one two-thousandth the mass of a hydrogen atom. Other experimental results, some by other scientists, suggested that the ratio of the charge to the mass of these particles had to be less than one-thousandth the ratio for charged hydrogen atoms. If they had momentum, that meant (in the physics of the time) that they had mass, suggesting that the rays were composed of tiny particles.
WILLIAM THOMSON CATHODE RAY EXPERIMENT SERIES
A series of experiments in which various objects were placed in the path of the rays showed that they also had momentum (they would cause a small paddle wheel to turn, for example). Thomson examined the nature of the rays' charge by bringing a positively charged and a negatively charged plate near the path of the rays, and observed that the rays were deflected toward the positive plate, suggesting they had negative charge. As an electric current passed from the cathode at one end of the tube to the anode at the other, raylike emanations were seen to proceed from the cathode to the anode. Thomson was studying what we now call cathode-ray tubes. The breakthroughs came in the late 1890s, when the British physicist J. Biography Nowadays we take for granted the existence of electrons, but this was not true just over 100 years ago, when the atom was thought to be a single unit that had no parts.


 0 kommentar(er)
0 kommentar(er)
